
The ASI Evolution® is the world’s first and only fully-automated system for nontreponemal RPR Syphilis testing FDA cleared for diagnostic, blood donor screening and cadaveric (Non-heart beating) tissue screening.
Arlington Scientific advocates the use of the RPR test (a nontreponemal test) that detects antibodies formed in response to damaged host cells as the primary way of screening donors. The RPR test indicates if a person has an active infection.
Among reported STI’s, syphilis continues to climb at an alarming rate. According to a new 2023 report by the CDC, “The United States is currently experiencing a syphilis epidemic, with sustained increases in primary and secondary syphilis.4 This increase affects almost every demographic nationally. These new record levels are the highest numbers seen in more than 70 years resulting in a 2,140% increase in syphilis.4
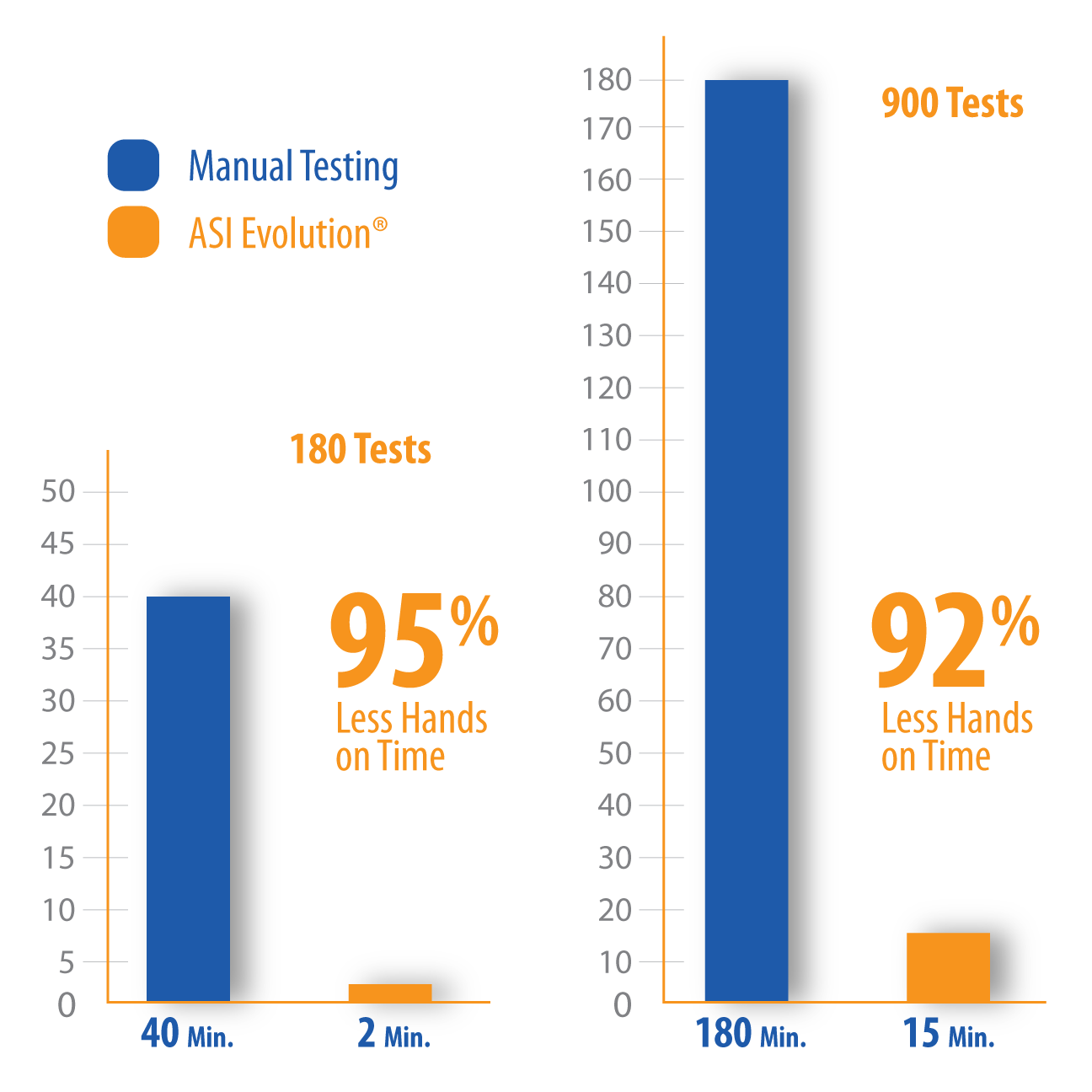
The ASI Evolution Automated (RPR, nontreponemal) Syphilis Analyzer, FDA cleared for diagnostic, blood donor and cadaveric tissue screening, is quickly becoming the standard for automated RPR screening. Now labs can utilize Lab Techs more efficiently and simplify their syphilis screening workload with the ASI Evolution.
The ASI Evolution reduces the amount of hands on time required to run an RPR test, freeing up valuable lab resources.
“The CDC continues to recommend the traditional screening algorithm using a nontreponemal test (e.g., RPR or VDRL), with reactive nontreponemal tests confirmed by treponemal testing.4
A 2015 CDC study determined that nontreponemal tests can detect infection up to 14 days earlier than treponemal tests.

The CDC continues to recommend the use of the RPR test as away of determining active infections. In the CDC’s 2023 draft of “Laboratory Recommendations for Syphilis Testing in the United States,” it states that two-thirds of labs or 63.1% of labs continue to use the RPR traditional algorithm. In a cost study, cited in this same draft guidance, it states that treponemal testing is three times more costly than nontreponemal testing based on the CMS (Centers for Medicare and Medicaid Services) fee schedule.



